physicist
Joseph John Thomson

| Personal information | ||
|---|---|---|
| Full name | Joseph John Thomson | |
| Birthdate | December 18, 1856 | |
| Date of death | August 30, 1940 | |
| Occupation | Physicist and mathematician | |
| Nationality | British | |
| Spouse | Rose Elisabeth Paget | |
| Parents | James Thomson and Emma Swindells | |
| Awards | Nobel Prize in Physics in 1906 | |
Joseph John Thomson Biography
Joseph John Thomson (December 18, 1856 – August 30, 1940) was born in Cheetham, Manchester, UK. British physicist and mathematician, awarded the Nobel Prize in Physics in 1906, for his contributions to the conduction of electricity through gases.
Thomson is recognized for his studies on the electron and the Plum Pudding Theory, also called Thomson’s atomic model, this theory delves into the atomic structure of the atom and the importance of electrons or negative charge. Son of Joseph James Thomson and Emma Swindells, he had as a brother Frederick Vernon Thomson. His father was a bookseller, who was always concerned about providing the best education for his children. He wanted Joseph J. Thomson to study engineering.
Education and career
From an early age, he was interested in mathematics and knowledge; and so in 1870, he entered Owens College, around 1876 he attended Trinity College, Cambridge. He obtained his degree as a Bachelor of Mathematics in 1883. After finishing his studies he began to work as a teacher at the same institution, where he taught mathematics and physics. In 1884, he was in charge of the Cavendish chair, and later, he was appointed Director of the Cavendish Laboratory at the University of Cambridge, a position he held from 1918 until he died in 1940.
While working at the Cavendish Laboratory he met physicist Niels Bohr, with whom he had a close relationship. At this point, he was a professor of the famous New Zealand physicist and chemist Ernest Rutherford. In 1890, he married Sir George E. Pagetm’s daughter, Rose Elisabeth.
Thomson served as professor of natural philosophy at the Royal Institution of Great Britain located in Great Britain. He held this position between 1905 and 1918. Upon leaving the institution, the position was taken by Thomas Young. At the same time, he was President of the Royal Society between 1915 and 1920. After being the chair at the Royal Institution of Great Britain, he returned to the Cavendish Laboratory of the University of Cambridge. Thomson passed away on August 30, 1940, in Cambridge. He was later buried in Westminster Abbey.
Thomson’s discoveries and works
Thomson began researching atomic structure while studying and teaching at the Cavendish Chair. Throughout his career he investigated cathode rays; While he was conducting various experiments with these, he discovered that electric fields could deflect the direction of cathode rays. After this discovery, he devoted himself entirely to investigating the cause of the deviation, the way it deviated, and their relationship to the charge and mass of the particles. In 1897 while conducting experiments with cathode-ray tubes, he discovered a new particle, which was lighter than hydrogen, this was called an electron. Thomson was the first scientist to identify subatomic particles.
After discovering the electron he focused on unraveling all the mysteries that surrounded this subatomic particle, for this he built a tool that would allow the analysis of the composition of the atom, this instrument was patented with the name of Mass Spectrometer. With this tool, Thomson found the relationship between the mass of the electron and the electric charge. Using this tool, he discovered that neon has two isotopes, which are neon-20 and neon-22.
Thomson’s Atomic Model
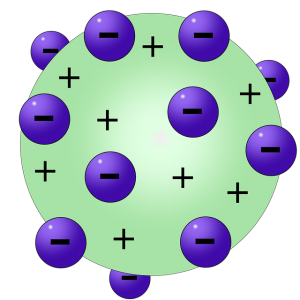
Thomson’s Atomic Model (Imagen de Vanessa Silvestre en Pixabay)
After several years of research, Thomson formulated the Plum Pudding Theory, also known as Thomson’s Atomic Model. This was a new model of atomic structure in which Thomson stated that electrons were like plums in a pudding made of positive matter. His model was wrong since it assumed that the electrons were mixed homogeneously with the positive charges, later it was shown that it was not that way.
After finishing his studies on the electron, he focused on electrical conduction; he carried out various experiments and studies on the passage of electricity through the interior of gases. In it, he studied, analyzed, and calculated what was the amount of electricity transferred by each atom, also, he calculated the number of molecules per cubic centimeter. This work earned him the Nobel Prize in Physics in 1906. Throughout his career as a physicist, he published numerous studies, among the most prominent are: The discharge of electricity through gases (1898), Conduction of Electricity Through Gases (1903), The Corpuscular Theory of Matter (1907), and The Electron in Chemistry (1923) and Recollections and Reflections (1936).

History
Robert Oppenheimer

Robert Oppenheimer Biography
Julius Robert Oppenheimer (April 22, 1904 – February 18, 1967) was a physicist widely acknowledged as the father of atomic bomb. He was born in New York City, United States. Oppenheimer is renowned for his pivotal role in the development of the Manhattan Project during World War II, which culminated in the creation of the first atomic bombs.
Early years
Robert Oppenheimer was an American theoretical physicist, scientist, and university professor. He was the son of German-born Julius S. Oppenheimer and artist Ella Friedman. Coming from a wealthy and educated family in New York, his father was a Jewish owner of a significant fortune amassed through his textile factory. This allowed Oppenheimer to enjoy certain comforts and attend the best schools in the city.
In this regard, he was educated at the Ethical Culture School in New York, where he excelled as the top student, with some teachers even asserting that he was better than many of them. Upon graduating from school, he enrolled at Harvard University, where he stood out in all areas, from chemistry to Eastern philosophy, Greek, and Latin, completing his studies at the age of 21.
Career
After some time, Robert Oppenheimer decided to delve into the world of physics, capitalizing on both his aptitude for the subject and its burgeoning prominence across Europe. He moved to England, where he worked with Ernest Rutherford and J. J. Thomson. Alongside these two great scientists, he felt somewhat inadequate, as he believed his performance did not meet the required standards. However, Oppenheimer did not give up.
With the passage of time, he learned from his mentors, and by 1925, feeling more adapted, he began researching atomic energy and secondary-atomic particle energy processes at the Cavendish Laboratory.
A year later, he was at the University of Göttingen, collaborating with Max Born to develop his classical contribution to molecular quantum theory, known in physics as the “Born-Oppenheimer method”. He returned to his home country to teach theoretical physics at the California Institute of Technology and the University of Berkeley between 1929 and 1942. Oppenheimer was a highly political person. Therefore, in the 1930s, he decided to align himself with communist students to support the Republic during the Spanish Civil War, but certain actions led to the demise of his sympathy for the Communist Party.
Contribution to the Manhattan Project

General Leslie Groves alongside Oppenheimer in the development of the Manhattan Project (Circa 1944)
The civil and political situation in Europe was becoming increasingly dangerous. By 1939, Albert Einstein and Leo Szilard warned the U.S. government about the dangers of nuclear energy falling into the hands of the Nazis. In response, President Franklin Roosevelt initiated the project for creating the atomic bomb, which was initially under military control, led by General Leslie Groves, an engineer known for overseeing construction projects, including the Pentagon. Later, Oppenheimer was brought on board to manage the administration of the project, which culminated in the construction of the atomic bomb by 1945.
Oppenheimer’s most significant contribution to the Manhattan Project was his leadership and effective coordination among diverse teams of scientists. His vision and problem-solving skills were crucial in overcoming the scientific and technological challenges involved in building an atomic bomb. He supervised the design and testing of the first atomic bombs, including the successful detonation of the plutonium bomb at the Trinity test site in New Mexico on July 16, 1945.
This American physicist had the support of many prominent figures in science, such as the great scientist Werner Heisenberg, Erwin Schrödinger, Max Born, Wolfgang Pauli, Paul Dirac, and Enrico Fermi, with whom he also developed close friendships. The work carried out by these scientists on quantum physics provided significant support for his own research. He gained valuable insights into quantum and relativistic physics, which kept him abreast of new scientific developments. He made contributions to the application of quantum theory to the concept of electron spin.
In his role as the director of the Institute for Advanced Study in Princeton, he fostered discussion and research in quantum and relativistic physics. In 1953, his past ties with the Communist Party led to certain defamation issues. As a result, he was called to a security hearing where the accusation was dismissed, but his access to military secrets was still prohibited. One of the institutions that attacked the scientist the most was the Federation of American Scientists. Oppenheimer was humiliated, and his communications were monitored. Nine years later, President John F. Kennedy, in an effort to rectify this mistake, awarded him the Enrico Fermi Award, granted by the Atomic Energy Commission, which he received from President Johnson himself.
“Now I Am Become Death, the Destroyer of Worlds”.
Oppenheimer, confessed to President Harry Truman, that he was not entirely comfortable with the fate of the atomic bomb. After some time, the Cold War erupted, and the Soviet Union announced its possession of an atomic weapon. In response, the United States decided to develop an even more powerful weapon. They approached Oppenheimer to lead the Atomic Energy Commission, but he declined the offer, resulting in his removal from the position. However, he remained the director at the Institute for Advanced Study, thanks to the support given by Einstein, Von Neumann, and Bohr.
After this event, his life took a different turn as he decided to distance himself from the laboratories and leaned towards writing about the studies conducted throughout his scientific career. Notable works include “Science and the Common Understanding” (1954) and a book related to electrodynamics (published posthumously in 1970). In the aftermath of the events in Japan and the grave consequences the atomic bomb had on humanity, he made several proposals aimed at internationally regulating the use of atomic energy to ensure peace.
He staunchly opposed the creation of the hydrogen bomb. However, despite his efforts and those of the General Advisory Committee of the Atomic Energy Commission, its development continued. Disheartened, Oppenheimer made the decision to retire from Princeton in 1966. A year later, on February 18, 1967, he passed away from throat cancer.
Filmography
Visual works inspired by Robert Oppenheimer:
- “Fat Man and Little Boy” (1989): Oppenheimer was portrayed by Dwight Schultz in this film.
- “One Day” (1989): The scientist was portrayed by David Strathairn.
- “Oppenheimer” (2023): The biography of Julius Robert Oppenheimer was brought to the screen in this movie, based on the biographical work “American Prometheus” by author Kai Bird. The character of Julius Robert Oppenheimer was played by Cillian Murphy and directed by Christopher Nolan.
physicist
Anders Celsius

Anders Celsius biography
Anders Celsius (November 27, 1701 – April 25, 1744) was born in Uppsala, Sweden. Physicist and astronomer, creator of the centesimal scale of the thermometer known as Grade Celsius (° C), which replaced the scale proposed by the German scientist, Daniel Gabriel Fahrenheit in 1724. Celsius, like many other scientists of his day, had a careful education that covered various fields. However, he focused fully on physics and astronomy, areas in which he excelled being considered one of the most prominent scientists of the 18th century. Throughout his academic career, he served as a professor of astronomy at the University of Uppsala. He was also one of the supervisors of the construction of the Uppsala Observatory, which he directed for several years.
He was born in a family belonging to the academic circle of the country. His father was Nils Celsius, an outstanding astronomer, a descendant of Magnus Celsius, a renowned mathematician and astronomer, who deciphered the runes of Staveless. His uncle Olof Celsius was the creator of a botanical school in Uppsala and a professor famous for his knowledge about mosses. On the maternal side, Celsius is related to Anders Spole, an astronomer and mathematician who served as a professor at Upsala University.
Career
After completing his studies he began to practice as a professor at the University of Uppsala (1730-1744) for 14 years. During this time, he conducted various investigations related to the field of astronomy. In the early years of the 1730s, he undertook a trip through Europe in which he visited the most outstanding astronomical observatories of the time, arriving to work with renowned astronomers. In 1733, he published a compilation of 316 observations of northern lights, in which he speculated about their relationship with magnetism.
Between 1736 and 1737 he was part of the group of researchers that accompanied the French astronomer Pierre Louis Maupertuis, on his journey through the northern region of Sweden where he sought to measure the length of the meridian near the pole, to compare it with the measurement made in Peru near to Ecuador. This research was known as the Lapland Expedition, which sought to demonstrate that Newton’s predictions about the flattening of the earth at the poles were correct, a conclusion they reached after the measurements. The calculations and conclusions of the expedition were included in La Figure de la Terre, a book published by Maupertuis in 1738.
For his participation in the expedition, Celsius was rewarded as an annual pension of 1,000 pounds, economic income that allowed him to invest in the construction of the Uppsala Observatory, which was one of the most modern of his time, after the opening he was appointed director of the observatory (1740). During the following years, he made various geographical measures used in the Swedish map. In the 1740s he carried out the studies in relation to the temperature scale by which he is known.
By 1742, he proposed to the Swedish academy a new way of measuring the temperature based on two established points: 0 indicated the boiling point of water and 100 represented the degree of freezing; which meant that as the heat increased the temperature dropped. This proposal would replace the scale created by Daniel Gabriel Fahrenheit in 1724, known as Fahrenheit Grade (° F), which ranged from 32 to 212 degrees.
Explained the operation of the scale, Celsius, created the centesimal scale that ranged from 0 to 100 degrees and invented the mercury thermometer. After three years, the scale was reversed by the Swedish scientist Karl von Linné, a modification with which it has been used since then. The scale of the Swedish scientist was called in the first years, Swedish thermometer, a term used by the scientific community of the time, however, since the 19th century it began to be called Celsius thermometer, in homage to its creator, it has also been known as Grade Celsius (° C). The following century this was replaced by the Kelvin scale (Kelvin K Grade), created in 1848 by William Thomson (Lord Kelvin).
The contributions of Celsius in the field of science are not reduced to scale, he was also the first scientist to raise the relationship between the phenomenon of the auroras and magnetism, also made numerous observations of this phenomenon that allowed his study years late. Another contribution of this scientist in the astronomical field was his studies on eclipses and stars, which included a detailed catalog of 300 stars and their system. Two years after the scale was created, the Swedish scientist contracted tuberculosis, a disease that deteriorated his health in a short time, passing away on April 25th, 1744, at the age of 43.
Doctor
Daniel Bernoulli

Daniel Bernoulli biography
Daniel Bernoulli (January 29, 1700 – March 17, 1782) mathematician, statistician, physicist, and physician. He was born in Groningen, Holland. His father, Johann Bernoulli, was a researcher who made important contributions to the early development of calculus. The family had to flee to the Netherlands due to the persecution of the Huguenots. After a brief period in Frankfurt, they settled in Basel, Switzerland. Bernoulli was always a very intelligent and curious young man. In high school, he achieved notable qualifications and mastered three languages, upon graduation he entered the university to study medicine and obtained his degree in 1721 thanks to his thesis on breathing where he assumed the mechanistic approach that prevailed at the time and was closer of his intellectual inclinations.
As soon as he graduated he tried to enter as a professor at the University of Basel but was rejected. Subsequently, Daniel was invited to work at the St. Petersburg Academy of Sciences, as a professor of mathematics. In this place, he met the great mathematician Leonhard Euler and then became his collaborator. He corresponded with the Prussian mathematician Christian Goldbach, most of the correspondence was about the lessons learned with his father, dazzled by the level of Bernoulli, decided to publish the letters written by Daniel.
From 1731 he began research on the problems of life and health from the statistics. Two years later he returned to Basel where he served as professor of anatomy, botany, philosophy, and physics. Simultaneously, he advanced important hydrodynamic studies, for Bernoulli this was one of the most important properties of fluid flow, pressure, density, and velocity.
From these studies arose The Bernoulli Principle or the Dynamic Theory of fluids. In his theory, he gave a masterly explanation about the pressure of the gas on the walls of a container. Because of the above, he obtained a remarkable amount of prizes and recognition between 1725 and 1749. He also obtained many more for his studies in astronomy, gravity, tides, magnetism, ocean currents and the behavior of a boat in the sea. It is notorious that he maintained a bad relationship with his father from 1734, the year in which both shared the annual prize of the Academy of Sciences of Paris.
Johann came to expel him from his home and also published a book called Hydraulica in which he tried to attribute the discoveries of his son in this matter. His popularity and intelligence earned him a place at the University of Basel, in the chair that his father had occupied. During this period as a professor, he published 86 papers and won 10 prizes from the Paris Academy of Sciences. Later he was a member of the Royal Society from May 3, 1750. He always thought a lot about students who could not access education due to lack of resources, for that reason he advanced the construction of a pension for the shelter of students without resources, This was maintained until the end of his days with his money. In addition, while he was rector of the University of Basel, in 1744 and 1756.
He was very committed to the development of the University. He made monetary donations on several occasions for laboratory equipment and acquisition of new titles in the Library. His prestige grew considerably both as a lecturer in Theoretical Physics and especially for his uncommon classes of Experimental Physics. Normally his conferences were attended by more than one hundred participants, from different corners of Europe.
He is considered one of the precursors of the kinetic theory of gases. He proposed a model of the structure of gases, in which he assumed that atoms in continuous motion collided with each other and with the walls of the container that contains them; that approach was the starting point of the kinetic theory of gases. Bernoulli also analyzed the problem of analyzing the errors in the observations. Bernoulli showed the insufficiency of the reasoning handled at that time and advised to use a method that can be considered an antecedent to the method of least squares reformulated later by Gauss.
At present, and based on the studies of his texts, it is noticed that he was able to find in the Mathematical Analysis the means to extract from the calculations all the details of the phenomena. Daniel Bernoulli suffered a cardiac arrest on March 17, 1782, in Basel, this episode did not allow him to stay alive. At his funeral he was dismissed by hundreds of colleagues and relatives at a solemn ceremony of the Academy of Sciences of Paris organized by the philosopher and geometer Marqués de Condorcet, who then served as Perpetual Secretary, he also read a funeral eulogy that collects the merits of his work and also his characteristics as a true man of science.